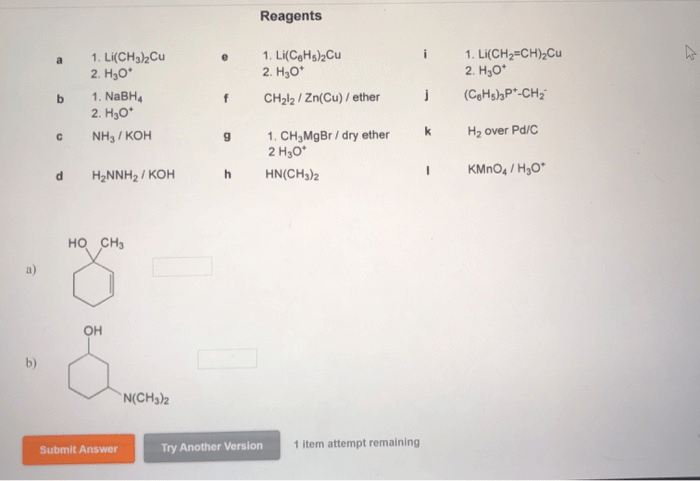
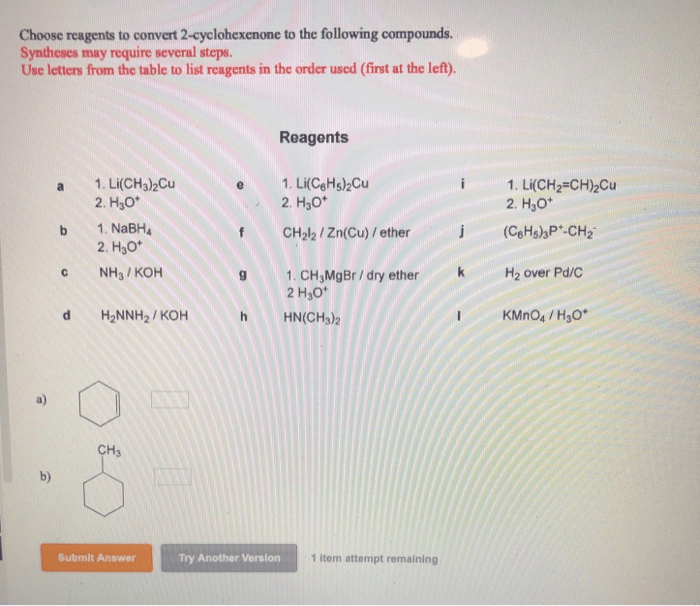
Choose reagents to convert 2-cyclohexenone to the following compounds – The conversion of 2-cyclohexenone to various compounds is a fundamental reaction in organic chemistry, offering a versatile platform for the synthesis of complex molecules. This comprehensive guide delves into the intricacies of reagent selection, exploring the mechanisms, selectivity, advantages, and disadvantages of different reagents for specific conversions.
From alcohols and epoxides to carboxylic acids and amines, we will navigate the diverse reaction pathways, examining the stereoselectivity and regioselectivity of each transformation. By understanding the nuances of reagent choice, chemists can optimize their synthetic strategies and achieve desired products with precision.
Choose Reagents to Convert 2-Cyclohexenone to Various Compounds: Choose Reagents To Convert 2-cyclohexenone To The Following Compounds
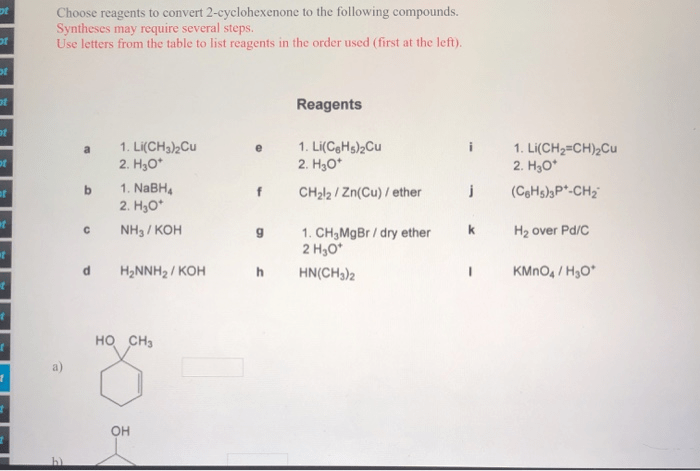
2-cyclohexenone is a versatile starting material that can be converted into a wide range of compounds. The choice of reagents used for these conversions depends on the desired product and the reaction conditions. This article provides an overview of the reagents and reaction conditions that can be used to convert 2-cyclohexenone to various compounds, including alcohols, epoxides, carboxylic acids, and amines.
Identify Reagents, Choose reagents to convert 2-cyclohexenone to the following compounds
- Sodium borohydride (NaBH4) : Reduces 2-cyclohexenone to 2-cyclohexen-1-ol, a primary alcohol.
- Lithium aluminum hydride (LiAlH4) : Reduces 2-cyclohexenone to 2-cyclohexanol, a secondary alcohol.
- Grignard reagents (RMgX): Add to 2-cyclohexenone to form tertiary alcohols.
- Peroxyacids (RCO3H) : Epoxidize 2-cyclohexenone to form epoxides.
- Potassium permanganate (KMnO4) : Oxidizes 2-cyclohexenone to adipic acid, a carboxylic acid.
- Sodium cyanide (NaCN): Adds to 2-cyclohexenone to form 2-cyclohexenecarbonitrile, which can be hydrolyzed to 2-cyclohexenecarboxylic acid.
- Ammonia (NH3) : Adds to 2-cyclohexenone to form 2-cyclohexenylamine, an amine.
Popular Questions
What factors should be considered when choosing reagents for 2-cyclohexenone conversion?
The choice of reagents depends on the desired product, the reaction mechanism, the selectivity required, and the availability and cost of the reagents.
How can I optimize the yield of a specific conversion?
Optimizing yield involves selecting reagents with high selectivity, using appropriate reaction conditions (temperature, solvent, etc.), and employing techniques such as protecting groups and catalysts.