Synthesize the following compound from benzene, a fundamental process in organic chemistry, unlocks a vast array of possibilities for creating complex and valuable molecules. This guide delves into the intricacies of benzene-based synthesis, empowering chemists with the knowledge to design and execute successful synthetic strategies.
From the importance of benzene as a starting material to the diverse methods employed for compound synthesis, this comprehensive resource provides a thorough understanding of the subject.
Introduction
Organic synthesis involves the deliberate construction of molecules from simpler starting materials. Synthesizing compounds from benzene, a ubiquitous aromatic hydrocarbon, is crucial for various industries and research endeavors.
Methods for Synthesizing Compounds from Benzene
Several methods are employed to synthesize compounds from benzene, each with its advantages and drawbacks:
- Electrophilic Aromatic Substitution: A versatile method involving the addition of an electrophile (e.g., NO 2+, SO 3H +) to the benzene ring.
- Nucleophilic Aromatic Substitution: A less common method that involves the substitution of an electrophile with a nucleophile (e.g., OH –, NH 2–).
- Radical Addition: A method that involves the addition of a radical species to the benzene ring, leading to the formation of alkylated or cycloalkylated products.
Step-by-Step Procedures
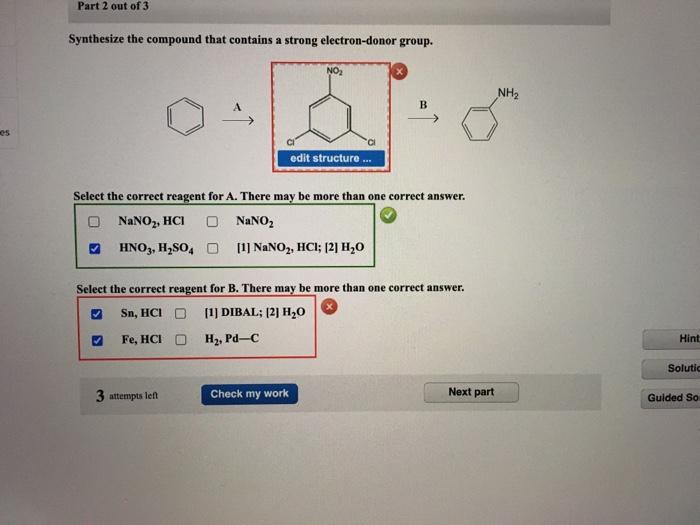
The specific steps involved in synthesizing compounds from benzene depend on the desired product and the chosen method.
For instance, to synthesize nitrobenzene via electrophilic aromatic substitution:
- Benzene is treated with concentrated nitric acid and sulfuric acid.
- The electrophile, NO 2+, is generated in situ.
- NO 2+attacks the benzene ring, forming a carbocation intermediate.
- The carbocation is stabilized by resonance and subsequently deprotonated to yield nitrobenzene.
Optimization and Characterization
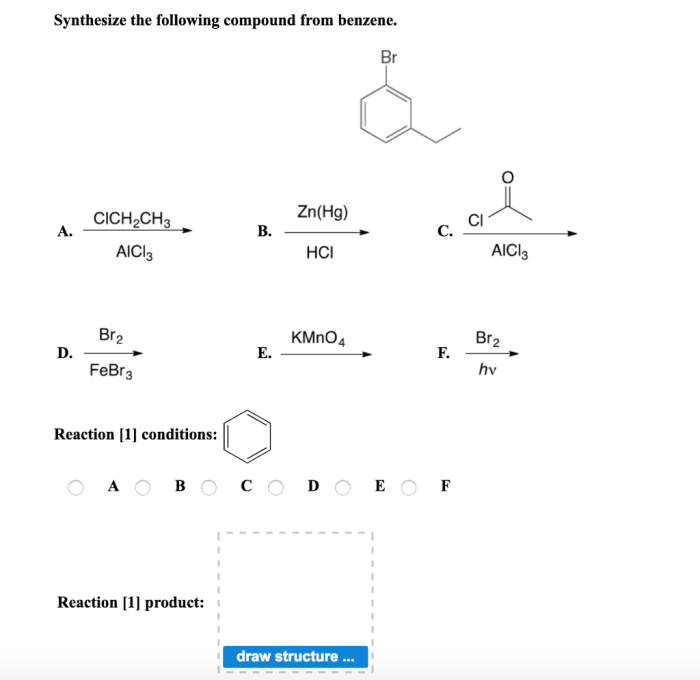
Optimizing the yield and purity of synthesized compounds involves adjusting reaction conditions, such as temperature, time, and catalyst.
Characterization techniques include:
- NMR spectroscopy
- Mass spectrometry
- Infrared spectroscopy
Applications of Synthesized Compounds
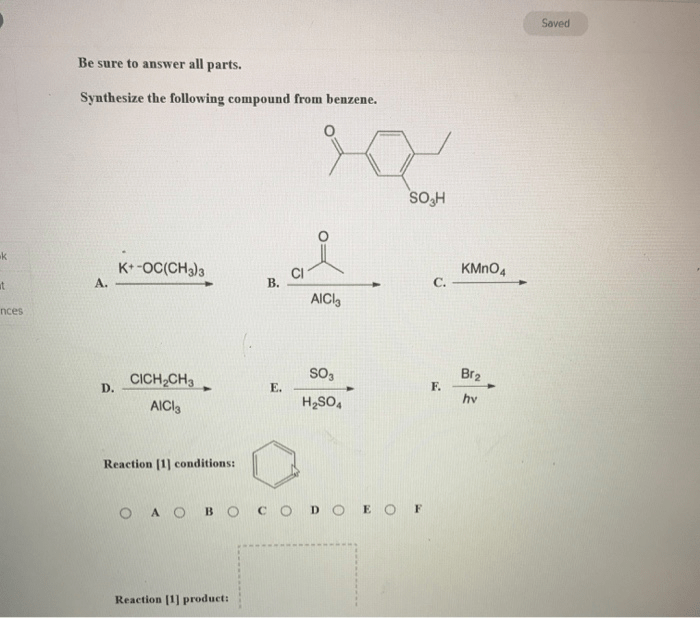
Compounds synthesized from benzene find widespread applications in:
- Pharmaceuticals (e.g., aspirin, ibuprofen)
- Materials science (e.g., plastics, polymers)
- Catalysis (e.g., Ziegler-Natta catalysts)
Safety Considerations
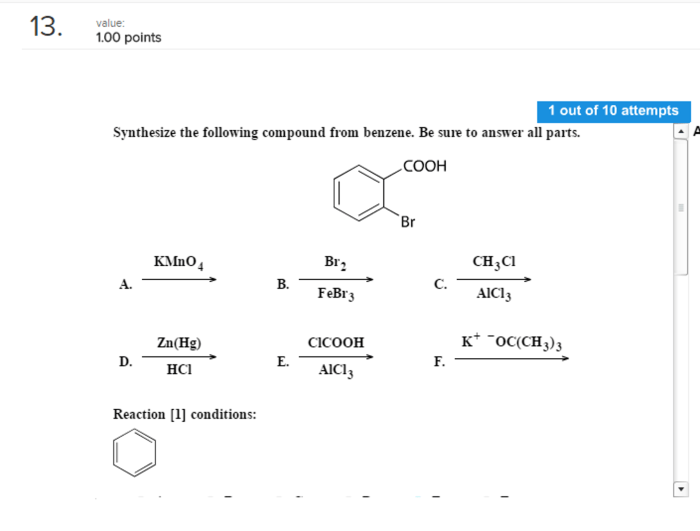
Benzene is a hazardous chemical that requires strict safety precautions:
- Proper ventilation and personal protective equipment are essential.
- Avoid prolonged exposure and skin contact.
- Dispose of waste materials appropriately.
Commonly Asked Questions: Synthesize The Following Compound From Benzene
What are the advantages of synthesizing compounds from benzene?
Benzene is a highly reactive and versatile starting material, allowing for the synthesis of a wide range of compounds. Its aromatic structure provides stability and facilitates various reactions, making it a preferred choice for complex molecule synthesis.
How can I optimize the yield and purity of synthesized compounds?
Optimization techniques such as temperature control, solvent selection, and reaction time optimization can enhance yield and purity. Additionally, purification methods like recrystallization, distillation, and chromatography are crucial for obtaining high-quality products.