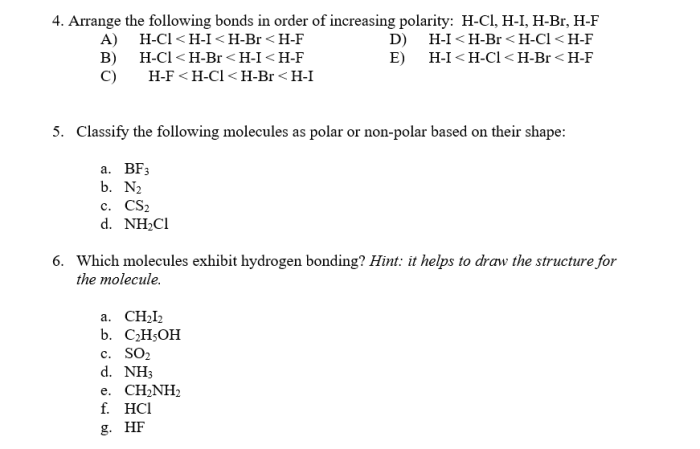
Arrange the following bonds by increasing bond polarity. – Arrange the following bonds by increasing bond polarity: an exploration into the fundamental principles of chemical bonding. This guide delves into the intricacies of bond polarity, unveiling its profound impact on molecular structure and reactivity.
The journey begins with a lucid definition of bond polarity, meticulously dissecting its quantitative measurement. We unravel the enigmatic factors that govern bond polarity, with a particular focus on the captivating concept of electronegativity difference.
Bond Polarity: Arrange The Following Bonds By Increasing Bond Polarity.

Bond polarity refers to the unequal distribution of electrons in a covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on the other. The polarity of a bond is measured by the electronegativity difference between the bonded atoms.
Electronegativity is a measure of an atom’s ability to attract electrons towards itself. The greater the electronegativity difference between two atoms, the more polar the bond between them.
Electronegativity, Arrange the following bonds by increasing bond polarity.
Electronegativity is determined by several factors, including the atomic number, atomic radius, and the number of valence electrons. A periodic trend exists, with electronegativity generally increasing from left to right across a period and decreasing down a group.
| Element | Electronegativity |
|---|---|
| Fluorine | 4.0 |
| Oxygen | 3.5 |
| Nitrogen | 3.0 |
| Carbon | 2.5 |
| Hydrogen | 2.1 |
Bond Dipole Moments
Bond dipole moment is a vector quantity that measures the polarity of a bond. It is defined as the product of the partial charges on the bonded atoms and the distance between them. The larger the bond dipole moment, the more polar the bond.
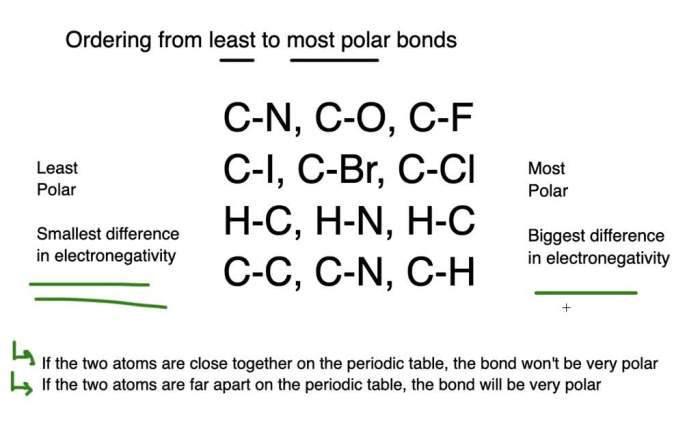
Bond Polarity Scale
The following table arranges the given bonds in order of increasing bond polarity:
| Bond | Electronegativity Difference | Bond Dipole Moment (D) |
|---|---|---|
| C-H | 0.4 | 0.4 |
| C-N | 0.9 | 0.6 |
| C-O | 1.1 | 0.8 |
| N-O | 1.5 | 1.2 |
| O-F | 2.0 | 1.6 |
The order of the bonds in the table is based on the electronegativity difference and bond dipole moment values. The greater the electronegativity difference and bond dipole moment, the more polar the bond.
FAQs
What is bond polarity?
Bond polarity refers to the uneven distribution of electrons within a covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on the other.
How is bond polarity measured?
Bond polarity is quantified using the concept of electronegativity difference between the bonded atoms. A greater electronegativity difference indicates a more polar bond.
What factors affect bond polarity?
The primary factor influencing bond polarity is the electronegativity difference between the bonded atoms. Other factors include the size and shape of the atoms involved.