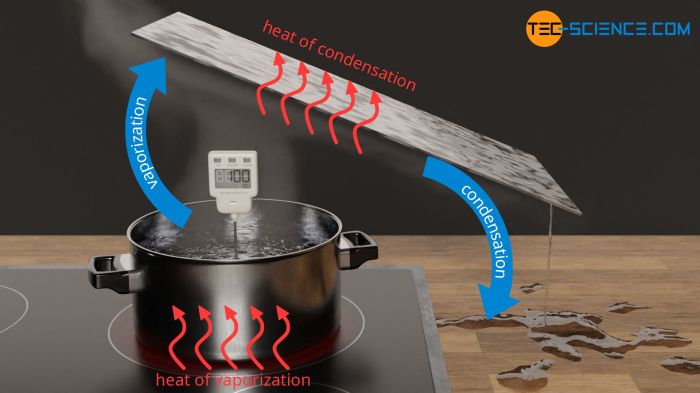
The heat of vaporization of water is 40.66 kJ/mol, a crucial thermodynamic property that plays a vital role in various industrial processes, heating and cooling systems, and everyday applications. This introductory paragraph delves into the significance of the heat of vaporization, setting the stage for a comprehensive exploration of its multifaceted nature.
Delving deeper into the topic, we will examine the experimental setup used to measure the heat of vaporization of water, unravel the formula and units involved in its calculation, and discuss factors that can influence the accuracy of the measurement.
Understanding the Concept of Heat of Vaporization: The Heat Of Vaporization Of Water Is 40.66 Kj/mol
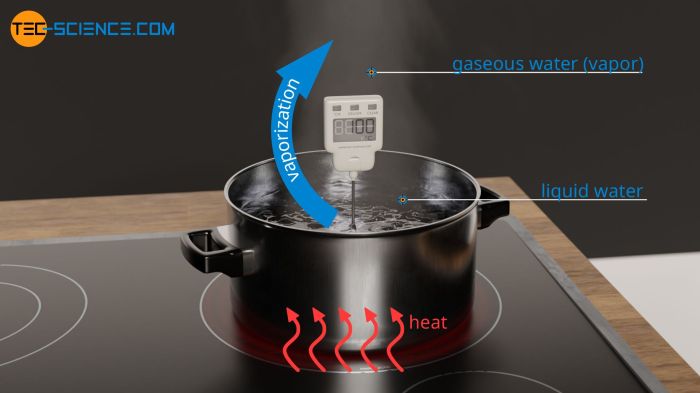
The heat of vaporization is the amount of energy required to convert a liquid into a gas at its boiling point. It is a thermodynamic property that measures the strength of the intermolecular forces holding the molecules together in the liquid phase.
The heat of vaporization is typically expressed in kilojoules per mole (kJ/mol) or calories per gram (cal/g).
The heat of vaporization is different from the heat of fusion, which is the energy required to convert a solid into a liquid. The heat of vaporization is typically much larger than the heat of fusion, reflecting the stronger intermolecular forces in the liquid phase compared to the solid phase.
Different substances have different heats of vaporization. For example, the heat of vaporization of water is 40.66 kJ/mol, while the heat of vaporization of ethanol is 38.6 kJ/mol.
Calculating the Heat of Vaporization of Water, The heat of vaporization of water is 40.66 kj/mol
The heat of vaporization of water can be measured using a variety of experimental techniques. One common method is to use a calorimeter. A calorimeter is a device that measures the amount of heat transferred between a system and its surroundings.
In a typical experiment, a known mass of water is placed in a calorimeter. The calorimeter is then heated, and the temperature of the water is measured. The heat of vaporization of water can be calculated using the following formula:
“`Q = mL“`where:
- Q is the heat of vaporization (kJ)
- m is the mass of water (g)
- L is the heat of vaporization of water (kJ/g)
The accuracy of the measurement of the heat of vaporization of water depends on a number of factors, including the accuracy of the calorimeter, the accuracy of the temperature measurement, and the purity of the water.
Applications of the Heat of Vaporization of Water
The heat of vaporization of water has a number of important applications in industry and everyday life.
In industry, the heat of vaporization of water is used in a variety of processes, including:
- Distillation: Distillation is a process used to separate liquids based on their boiling points. The heat of vaporization of water is used to vaporize the more volatile liquid, which is then condensed and collected.
- Evaporation: Evaporation is a process used to remove water from a substance. The heat of vaporization of water is used to vaporize the water, which is then removed from the substance.
In everyday life, the heat of vaporization of water is used in a variety of applications, including:
- Heating and cooling systems: The heat of vaporization of water is used in heating and cooling systems to transfer heat from one place to another.
- Refrigeration: The heat of vaporization of water is used in refrigeration systems to remove heat from a space.
Comparison with Other Substances
The heat of vaporization of water is higher than the heat of vaporization of most other liquids. This is due to the strong hydrogen bonding between water molecules.
The following table compares the heats of vaporization of different liquids:
| Liquid | Heat of Vaporization (kJ/mol) |
|---|---|
| Water | 40.66 |
| Ethanol | 38.6 |
| Methanol | 35.3 |
| Acetone | 29.1 |
The heat of vaporization of a liquid can be used to identify and differentiate between different substances.
Advanced Applications and Research
The heat of vaporization of water is also used in a number of advanced applications, including:
- Heat pumps: Heat pumps are devices that use the heat of vaporization of water to transfer heat from one place to another. Heat pumps are used to heat and cool buildings.
- Thermal energy storage systems: Thermal energy storage systems use the heat of vaporization of water to store thermal energy. Thermal energy storage systems are used to store energy from renewable sources, such as solar and wind energy.
Ongoing research is focused on developing new and innovative applications for the heat of vaporization of water. These applications include using the heat of vaporization of water to generate electricity and to desalinate water.
General Inquiries
What is the difference between heat of vaporization and heat of fusion?
The heat of vaporization refers to the energy required to convert a substance from a liquid to a gas, while the heat of fusion refers to the energy required to convert a substance from a solid to a liquid.
How does the heat of vaporization affect the boiling point of a liquid?
The heat of vaporization is directly proportional to the boiling point of a liquid. Liquids with higher heats of vaporization have higher boiling points.
What are some practical applications of the heat of vaporization?
The heat of vaporization is utilized in various applications, including distillation, evaporation, heating and cooling systems, and thermal energy storage.